AIPMT 2015 Chemistry Online Model Test Paper- 4 Practice MCQs Multiple Choice Questions Fully Solved with Answer Key Free
AIPMT All India Pre Medical Test is conducted by CBSE in compliance with the directives of the Hon'ble Supreme Court of India for admissions to 15% seats of Medical/ Dental colleges of India. Some other State Govt university or institutions also use AIPMT merit list for admission in the Medical Colleges/Dental Colleges against seats under their control. Here we have published model test paper for Chemistry for practice purpose with objective type MCQs multiple choice questions based on AIPMT previous years question papers.
AIPMT 2015 Chemistry Model Test Paper - 4
Q.1. In a first-order reaction A-> B, if k is rate constant and initial concentration of the reactant A is 0.5 M then the half-life is :
1) In 2/K
2) 0.693/0.5K
3) log2/K
4) log2/K√(0.5)
2) 0.693/0.5K
3) log2/K
4) log2/K√(0.5)
Q.2. The reaction of hydrogen and iodine monochloride is given as:
H2(g) + 2ICl(g) -> 2HCl(g) + I2(g)
This reaction is of first order with respect to H2(g) and ICl(g) , following mechanisms were proposed :
Mechanism A : H2(g) + 2ICl(g) -> 2HCl(g) + I2(g)
Mechanism B : H2(g) + ICl(g) -> HCl(g) +HI2(g): slow HI(g)+ ICl(g) -> HCl(g) + I2(g); fast
Which of the above mechanism (s) can be consistent with the given information about the reaction
1) A Only
2) B Only
3) 1 and 2 both
4) Neither 1 nor 2
2) B Only
3) 1 and 2 both
4) Neither 1 nor 2
Q.3. If 60% of a first order reaction was completed in 60 minutes, 50% of the same reaction would be completed in approximately :
1) 40 Minutes
2) 50 Minutes
3) 45 Minutes
4) 60 Minutes
2) 50 Minutes
3) 45 Minutes
4) 60 Minutes
Q.4. The equilibrium constant of the reaction :
C u(s) + 2Ag+ (aq) -> Cu2+ (aq) + 2Ag(s); E0 = 0.46V att 198K is :
1) 4.0 * 1015
2) 2.4 * 1010
3) 2.0 * 1010
4) 4.0 * 1010
2) 2.4 * 1010
3) 2.0 * 1010
4) 4.0 * 1010
Q.5. 0.5 molal aqueous solution of a weak acid (HX) is 20% ionized. If Kf for water is 1.86 K kg mol sup-1 , the lowering in freezing point of the solution is
1) - 0.56 K
2) - 1.12 K
3) 0.56 K
4) 1.12 K
2) - 1.12 K
3) 0.56 K
4) 1.12 K
Q.6. The measurement of the electron position is associated with an uncertainty in momentum, which is equal to 1 × 10-18 g cm s-1. The uncertainty in electron velocity is, (mass of an electron is 9 × 10-28 g)
1) 1 × 1011 cm s-1
2) 1 × 109 cm s-1
3) 1 × 106 cm s-1
4) 1 × 105 cm s-1
2) 1 × 109 cm s-1
3) 1 × 106 cm s-1
4) 1 × 105 cm s-1
Q.7. Which of the following complex ion is not expected to absorb visible light?
1) [Ni(CN)4]2-
2) [Cr(NH3)6]3+
3) [Fe(H2O6)6]2+
4) [Ni(H2O)6]2+
2) [Cr(NH3)6]3+
3) [Fe(H2O6)6]2+
4) [Ni(H2O)6]2+
Q.8. The bromination of acetone that occurs in acid solution is represented by this equation
CH3COCH3 (aq) + Br2 (aq) -> CH3COCH2Br (aq) + H+ (aq) + Br- (aq)
These kinetic data were obtained for given reaction concentrations
Initial Concentration, M
| [CH3COCH3] | [Br2] | [H+] |
|---|---|---|
| 0.30 | 0.05 | 0.05 |
| 0.30 | 0.10 | 0.05 |
| 0.30 | 0.10 | 0.10 |
| 0.40 | 0.05 | 0.20 |
5.7 * 10-5 5.7 * 10-5 1.2 * 10-4 3.1 * 10-4
Based on the data, the rate equation is
1) Rate = k[CH3COCH3][Br2][H+]
2) Rate = k[CH3COCH3][H+]
3) Rate = k[CH=COCH3][Br2]
4) Rate = k[CH3COCH3][Br2][H+]2
2) Rate = k[CH3COCH3][H+]
3) Rate = k[CH=COCH3][Br2]
4) Rate = k[CH3COCH3][Br2][H+]2
Q.9. What volume of oxygen gas (O2) measured at 0°C and 1 atm, is needed to burn completely 1 L of propane gas (C3H8) measured under the same conditions?
1) 10L
2) 7L
3) 6L
4) 5L
2) 7L
3) 6L
4) 5L
Q.10. Bond dissociation enthalpy of H2, Cl2 and HCl are 434, 242 and 431 kJmol-1 respectively. Enthalpy of formation of HCl is
1) 245 kJmol-1
2) 93 kJmol-1
3) -245 kJmol-1
4) -93 kJmol-1
2) 93 kJmol-1
3) -245 kJmol-1
4) -93 kJmol-1
Q.11. Which one of the elements with the following outer orbital configurations may exhibit the largest number of oxidation states?
1) 3d54s1
2) 3d54s2
3) 3d24s2
4) 3d34s2
2) 3d54s2
3) 3d24s2
4) 3d34s2
Q.12. The stability of + 1 oxidation state increases in the sequence:
1) Tl < In < Ga < Al
2) In < Tl < Ga < Al
3) Ga < In < Al < Tl
4) Al < Ga < In < Tl
2) In < Tl < Ga < Al
3) Ga < In < Al < Tl
4) Al < Ga < In < Tl
Q.13. Given:
(i) Cu2+ + 2e- -> Cu, Eo = 0.337 V
(ii) Cu2+ + e- -> Cu+, Eo = 0.153 V
Electrode potential, Eo for the reaction, Cu+ + e- -> Cu, will be:
1) 0.90 V
2) 0.30 V
3) 0.38 V
4) 0.52 V
2) 0.30 V
3) 0.38 V
4) 0.52 V
Q.14. For the reaction, N2 + 3H2 -> 2 NH3, if d[NH3]/dt = 2 * 10-4 mol L-1s-1 the value of -d[H2]/dt would br:,
1) 4 * 10-4 mol L-1s-1
2) 6 * 10-4 mol L-1s-1
3) 1 * 10-4 mol L-1s-1
4) 3 * 10-4 mol L-1s-1
2) 6 * 10-4 mol L-1s-1
3) 1 * 10-4 mol L-1s-1
4) 3 * 10-4 mol L-1s-1
Q.15. Consider the following reaction,
1) CH3CH2-O-CH2-CH3
2) CH3-CH2-O-SO3H
3) CH3CH2OH
4) CH2=CH2
2) CH3-CH2-O-SO3H
3) CH3CH2OH
4) CH2=CH2
Q.16. In which of the following pairs of molecules/ions, the central atoms have sp2 hybridization?
1) NO2- and NH3
2) NH3 and NO2-
3) NH2- and H2O
4) BF3 and NH2-
2) NH3 and NO2-
3) NH2- and H2O
4) BF3 and NH2-
Q.17. Liquid hydrocarbons can be converted to a mixture of gaseous hydrocarbons by:
1) Oxidation
2) Cracking
3) Distillation under reduced pressure
4) Hydrolysis
2) Cracking
3) Distillation under reduced pressure
4) Hydrolysis
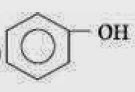
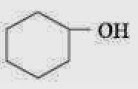
Q.18. Given are cyclohexanol (I), acetic acid (II), 2, 4, 6-trinitrophenol (III) and phenol (IV). In these the order of decreasing acidic character will be:
1) III > II > IV> I
2) II > III > I > IV
3) II > III > IV > I
4) III > IV > II > I
2) II > III > I > IV
3) II > III > IV > I
4) III > IV > II > I
Q.19. Which one of the following compounds has the most acidic nature?
Q.20. The reaction of toluene with Cl2 in presence of FeCl3
A) X = Benzal chloride, Y = o-chlorotoluene
B) X = m-chlorotoluene, Y = p-chlorotoluene
C) X = o-and p-chlorotoluene, Y = Trichloromethyl benzene
D) X = Benzyl chloride, Y = m-chlorotoluene
B) X = m-chlorotoluene, Y = p-chlorotoluene
C) X = o-and p-chlorotoluene, Y = Trichloromethyl benzene
D) X = Benzyl chloride, Y = m-chlorotoluene
Answer Key:
Q.1-
|
1
|
Q.2-
|
2
|
Q.3-
|
3
|
Q.4-
|
1
|
Q.5-
|
4
|
|---|---|---|---|---|---|---|---|---|---|
Q.6-
|
2
|
Q.7-
|
1
|
Q.8-
|
2
|
Q.9-
|
4
|
Q.10-
|
4 |
| Q.11- | 2 | Q.12- | 4 | Q.13- | 4 | Q.14- | 4 | Q.15- | 3 |
Q.16-
|
2
|
Q.17-
|
2
|
Q.18-
|
1
|
Q.19-
|
2 |
Q.20-
|
3
|
We have tried our best to present important questions with correct answer key. Any suggestion or update about any erroneous entry or answer is always appreciable. Please comment your score or any suggestion in comment section below.
Recommended Posts:-
- AIPMT Biology Model Practice Test Papers
- AIPMT Chemistry Model Practice Test Papers
- AIPMT Previous Years Question Papers with Complete Solution and Answer Key
- AIPMT Chemistry Best Books Objective Type.
- AIPMT Physics Best Books Objective Type.
- AIPMT Biology Best Books Objective Type.
- AIPMT Previous Years Solved Question Papers Best Books Objective Type.



Comments
Post a Comment