AIPMT 2015 Chemistry Online Model Test Paper- 5 Practice MCQs Multiple Choice Questions Fully Solved with Answer Key Free
AIPMT All India Pre Medical Test is conducted by CBSE in compliance with the directives of the Hon'ble Supreme Court of India for admissions to 15% seats of Medical/ Dental colleges of India. Some other State Govt university or institutions also use AIPMT merit list for admission in the Medical Colleges/Dental Colleges against seats under their control. Here we have published model test paper for Chemistry for practice purpose with objective type MCQs multiple choice questions based on AIPMT previous years question papers.
AIPMT 2015 Chemistry Model Test Paper - 5
Q.1. Two gases A and B having the same volume diffuse through a porous partition in 20 and 10 seconds respectively. The molecular mass of A is 49 u. Molecular mass of B will be
1) 25.00 u
2) 50.00 u
3) 12.25 u
4) 6.50 u
2) 50.00 u
3) 12.25 u
4) 6.50 u
Q.2. Which of the following is correct option for free expansion of an ideal gas under adiabatic condition?
1) q = 0, ΔT < 0, w not equal to 0
2) q = 0, ΔT not equal to 0, w = 0
3) q not equal to 0, ΔT = 0, w = 0
4) q = 0, ΔT = 0, w = 0
2) q = 0, ΔT not equal to 0, w = 0
3) q not equal to 0, ΔT = 0, w = 0
4) q = 0, ΔT = 0, w = 0
Q.3. For the reaction N2 (g) + O2 (g) ⇔ 2NO (g), the equilibrium constant is K1. The equilibrium constant is K2 for the reaction 2NO (g) + O2 (g) ⇔ 2NO2 (g). What is K for the reaction NO2(g) ⇔ 1/2N2 (g) + O2 (g)
1) 1/ (k1k2)
2) 1/ (2k1k2)
3) 1/ (4k1k2)
4) [1/ (k1k2)]1/2
2) 1/ (2k1k2)
3) 1/ (4k1k2)
4) [1/ (k1k2)]1/2
Q.4. If x is amount of adsorbate and m is amount of adsorbent, which of the following relations is not related to adsorption process?
1) X/m = p * T
2) X/m = f(p) at constant T
3) X/m = f(T) at constant p
4) p = f(T)at constant (X/m)
2) X/m = f(p) at constant T
3) X/m = f(T) at constant p
4) p = f(T)at constant (X/m)
Q.5. If the enthalpy change for the transition of liquid water to steam is 30 kJ mol-1at 27°C, the entropy change for the process would be
1) 100 J mol-1K-1
2) 10 J mol-1K-1
3) 1.0 J mol-1K-1
4) 0.1 J mol-1K-1
2) 10 J mol-1K-1
3) 1.0 J mol-1K-1
4) 0.1 J mol-1K-1
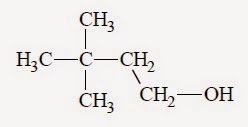
Q.6. In the following reaction:
The major product is
Q.7. The enthalpy of fusion of water is 1.435 kcal/mol. The molar entropy change for the melting of ice at 0°C
1) 0.526 cal/(mol K)
2) 10.52 cal / (mol K)
3) 21.04 cal / (mol K)
4) 5.260 cal/(mol K)
2) 10.52 cal / (mol K)
3) 21.04 cal / (mol K)
4) 5.260 cal/(mol K)
Q.8. Limiting molar conductivity of NH4OH (i.e. ^m(NH4OH) ) is equal to:
1) ^m(NH4Cl) + ^m(NH4OH) - ^m(NaCl)
2) ^m(NH4Cl) + ^m(NaCl) - ^m(NaOH)
3) ^m(NaOH) + ^m(NaCl) - ^m(NH4Cl)
4) ^m(NH4OH) + ^m(NH4Cl) -^m(HCl)
2) ^m(NH4Cl) + ^m(NaCl) - ^m(NaOH)
3) ^m(NaOH) + ^m(NaCl) - ^m(NH4Cl)
4) ^m(NH4OH) + ^m(NH4Cl) -^m(HCl)
Q.9. Which one of the following is a mineral of iron?
1) Magnetite
2) Malachite
3) Cassiterite
4) Pyrolusite
2) Malachite
3) Cassiterite
4) Pyrolusite
Q.10. In Freundlich Adsorption isotherm, the value of 1/n is:
1) 1 in case of chemisorption
2) between 0 and 1 in all cases
3) between 2 and 4 in all cases
4) 1 in case of physical adsorption
2) between 0 and 1 in all cases
3) between 2 and 4 in all cases
4) 1 in case of physical adsorption
Q.11. At 25ºC molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm–1 cm2 mol–1 and at infinite dilution its molar conductance is 238 ohm–1 cm2 mol–1. The degree of ionisation of ammonium hydroxide at the same concentration and temperature is -
1) 2.080 %
2) 20.800 %
3) 4.008 %
4) 40.800 %
2) 20.800 %
3) 4.008 %
4) 40.800 %
Q.12. Based on equation E = –2.178 × 10–18 J, [Z2/n2] certain conclusions are written. Which of them is not correct ?
1) The negative sign in equation simply means that the energy of electron bound to the nucleus is lower than it would be if the electrons were at the infinite distance from the nucleus.
2) Larger the value of n, the larger is the orbit radius.
3) Equation can be used to calculate the change in energy when the electron changes orbit.
4) For n = 1, the electron has a more negative energy that it does for n = 6 which means that the electron is more loosely bound in the smallest allowed orbit.
2) Larger the value of n, the larger is the orbit radius.
3) Equation can be used to calculate the change in energy when the electron changes orbit.
4) For n = 1, the electron has a more negative energy that it does for n = 6 which means that the electron is more loosely bound in the smallest allowed orbit.
Q.13. A button cell used in watches functions as following
Zn(s) + Ag2O(s) + H2O(l) 2Ag(s) + Zn2+ (aq) + 2OH– (aq)
If half cell potentials are
Zn2+ (aq) + 2e– → Zn(s) ; Eº = –0.76 V
Ag2O(s) + H2O(l) + 2e– → 2Ag(s) + 2OH– (aq), Eº = 0.34 V
The cell potential will be -
1) 1.10
2) 0.42
3) 0.84
4) 1.34
2) 0.42
3) 0.84
4) 1.34
Q.14. How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3 ? The concentrated acid is 70 % HNO3.
1) 45.0 g conc. HNO3
2) 90.0 g conc. HNO3
3) 70.0 g conc. HNO3
4) 54.0 g conc. HNO3
2) 90.0 g conc. HNO3
3) 70.0 g conc. HNO3
4) 54.0 g conc. HNO3
Q.15. The number of carbon atoms per unit cell of diamond unit cell is -
1) 4
2) 8
3) 6
4) 1
2) 8
3) 6
4) 1
Q.16. Which one of the following is not a common component of Photochemical Smog?
1) Peroxyacetyl nitrate
2) Chlorofluorocarbons
3) Ozone
4) Acrolein
2) Chlorofluorocarbons
3) Ozone
4) Acrolein
Q.17. For the reaction, X2O4(l) ⎯→ 2XO2(g) ΔU = 2.1 kcal, ΔS = 20 cal K–1 at 300 K Hence, ΔG is
1) 9.3 kcal
2) - 9.3 kcal
3) 2.7 kcal
4) -2.7 kcal
2) - 9.3 kcal
3) 2.7 kcal
4) -2.7 kcal
Q.18. Which one of the following species has plane triangular shape?
1) NO2-
2) CO2
3) N2
4) NO3-
2) CO2
3) N2
4) NO3-
Q.19. Which of the following organic compounds polymerizes to form the polyester Dacron?
1) Terephthalic acid and ethylene glycol
2) Benzoic acid and para HO — (C6H4) — OH
3) Propylene and para HO — (C6H4) — OH
4) Benzioc acid and ethanol
2) Benzoic acid and para HO — (C6H4) — OH
3) Propylene and para HO — (C6H4) — OH
4) Benzioc acid and ethanol
Q.20. Among the following complexes the one which shows Zero crystal field stabilization energy (CFSE) is
A) [Co(H2O)6]2+
B) [Co(H2O)6]3+
C) [Mn(H2O)6]3+
D) [Fe(H2O)6]3+
B) [Co(H2O)6]3+
C) [Mn(H2O)6]3+
D) [Fe(H2O)6]3+
Answer Key:
Q.1-
|
3
|
Q.2-
|
4
|
Q.3-
|
4
|
Q.4-
|
1
|
Q.5-
|
1
|
|---|---|---|---|---|---|---|---|---|---|
Q.6-
|
2
|
Q.7-
|
4
|
Q.8-
|
1
|
Q.9-
|
1
|
Q.10-
|
2 |
| Q.11- | 3 | Q.12- | 4 | Q.13- | 1 | Q.14- | 1 | Q.15- | 2 |
Q.16-
|
2
|
Q.17-
|
4
|
Q.18-
|
4
|
Q.19-
|
1 |
Q.20-
|
4
|
We have tried our best to present important questions with correct answer key. Any suggestion or update about any erroneous entry or answer is always appreciable. Please comment your score or any suggestion in comment section below.
Recommended Posts:-
- AIPMT Biology Model Practice Test Papers
- AIPMT Chemistry Model Practice Test Papers
- AIPMT Previous Years Question Papers with Complete Solution and Answer Key
- AIPMT Chemistry Best Books Objective Type.
- AIPMT Physics Best Books Objective Type.
- AIPMT Biology Best Books Objective Type.
- AIPMT Previous Years Solved Question Papers Best Books Objective Type.




Comments
Post a Comment